An Ionic Crystal Lattice Could Best Be Described as
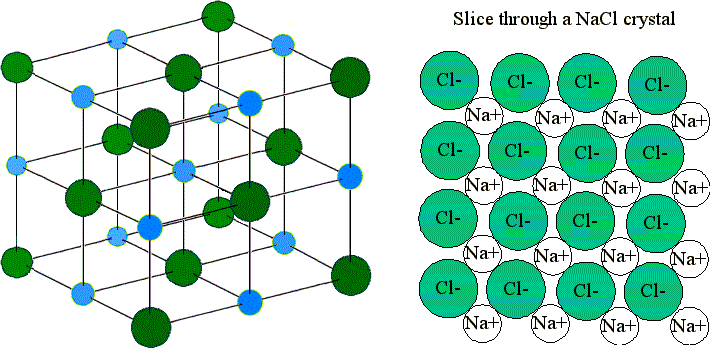
The easiest way to picture such an array is to arrange one layer of spheres and then place successive layers over it. Table salt is a good example of an ionic lattice.
9 2 Ionic Bonding And Lattice Energy Chemistry Libretexts
Silver halides are ionic crystals consisting of a regular cubic lattice of Ag and halide ions together with a small proportion of defects such as Ag ions that have been displaced from their regular lattice position to another interstitial position the Ag ions are much smaller than the halide ions and the corresponding vacancy in the lattice.

. These electric charges create an electromagnetic field and this field determines the properties of substances having an ionic lattice. Which of these best describes an ionic bond. Which compound has the highest melting point.
Which statement about crystal lattice energy is best supported by the information in the table. Which statement about crystal lattice energy is best supported by the information in the table. Well-known examples of ionic lattices are sodium chloride potassium permanganate borax sodium borate and copperII sulfate.
The lattice energy increases as cations get smaller as shown by LiF and KF. An ionic lattice has the opposite electric charge of ions. Which of these best describes an ionic bond.
A force that holds two oppositely charged ions together. In an ionic solid the ions are packed together into a repeating array called a crystal lattice. Which chemical species can easily form an ionic bond with a.
They exhibit strong absorption of infrared radiation and have planes along which they cleave easily. Simplest repeating unit of a face-centered cubic crystal. The Ionic Lattice If a crystal is formed of ions the compound can be described as an ionic lattice.
Silver halides are ionic crystals consisting of a regular cubic lattice of Ag and halide ions together with a small proportion of defects such as Ag ions that have been displaced from their regular lattice position to another interstitial position the Ag ions are much smaller than the halide ions and the corresponding vacancy in the lattice. XXX an attraction that occurs between two nonmetals. Although the lattice itself is fairly rigid.
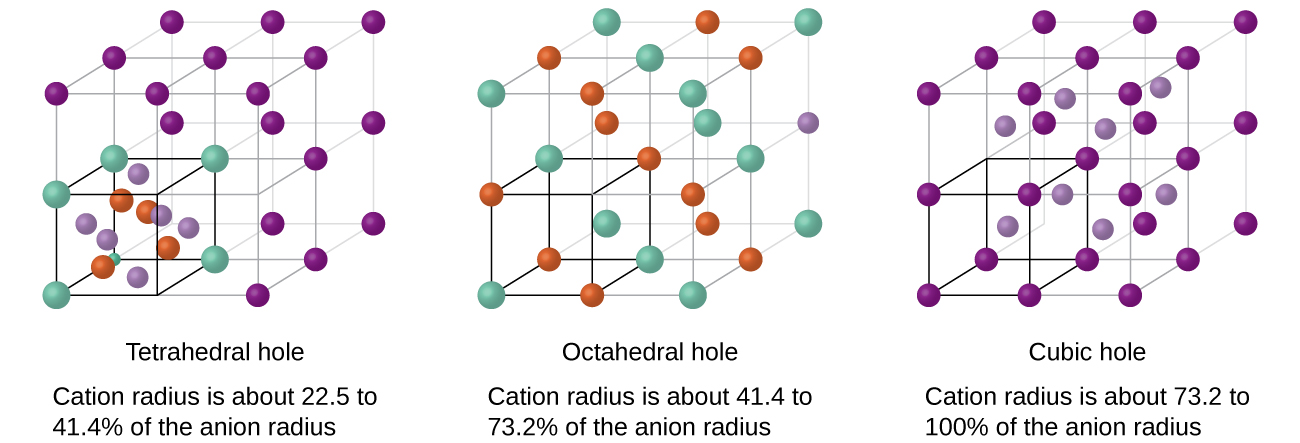
The concept of crystal packing assumes that the ions are hard spheres. It is a cube containing lattice points at each corner and in the center of each face hexagonal closest packing HCP crystalline structure in which close packed layers of atoms or ions are stacked as a series of two alternating layers of different relative orientations AB hole. The following properties are all characteristics of ionic.
The lattice energy increases as cations get smaller as shown by LiF and KF. A regular three-dimensional geometric arrangement of atoms molecules or ions in a crystal. Refractoriness hardness density and the ability to conduct electricity.
Although the lattice itself is fairly rigid. A class of crystal consisting of a lattice of ions held together by electrostatic interactions.

Crystal Lattice Structure Formation Expii
10 6 Lattice Structures In Crystalline Solids Chemistry

Comments
Post a Comment